Project Description
Poly (lactic acid) (PLA) is a semi – crystalline biodegradable, recyclable and compostable polymer. The advantages of PLA include eco-friendly (derived from renewable resources e.g. corn, wheat, or rice), biocompatibility (no toxic and no arcinogenic effect in human tissue), processability (can be processed by injection molding, film extrusion, blow molding, thermoforming, fiber spinning, and film forming) and energy savings-during the production (less energy 25–55% is required than other petroleum-based polymers). However, there are some limitations of application such as its poor toughness, slow degradation rate, hydrophobicity and lack of reactive side-chain groups. These disadvantages would be improved by copolymeization lactic acid with other polymers or comonomers, blending with flexible polymers or adding the plasticizers. The addition of plasticizers is the method that fast and inexpensive. It can be used to prepare a wide range of physical properties of the polymer which greatly broaden their applications.
In this study, the attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) is used to investigate the specific intermolecular interaction of the blends with different amounts and concentrations of fatty acids; lauric acid, palmitic acid and stearic acid.
Objectives
- To compare the chemical structures of poly(lactic acid)/poly(butylene succinate) blends containing fatty acids
- To investigate the interaction between poly(lactic acid)/poly(butylene succinate) blends containing fatty acids
- To compare the interaction of the blends prepared from film casting and melt blending method.
Experimental Section

Designations and compositions of PLA/PBS (90:10) – Fatty acids.
The blends prepared by film casting method.
The PLA and PBS were dried in a vacuum oven at 70 oC for 24 hours prior to use. The PLA/PBS blends of 90/10 by weight containing different types of fatty acids (lauric acid, succinic acid or palmitic acid) and different amounts of fatty acids (0, 2 and 4% by total weight of polymer) were dissolved in chloroform to obtain the mixture concentration 10% w/v. The mixtures were then left in fume hood to evaporate solvent overnight and dried in vacuum oven until weight constant obtained. Table 1 shows the composition of polymer blend samples and their designations.
The blends prepared by melt blending method.
The PLA and PBS were dried in a vacuum oven at 70 oC for 24 hours prior to use. The blends prepared by melt blending method were prepared with the same composition as the blends prepared by film casting method. The mixtures were melt- blended in a glass container using at 170 oC. The mixtures were then cooled down to room temperature and cut into small pieces before further characterization.
Results
1. Infrared spectra of the C=O absorption in the PLA/PBS blends
For the PLA/PBS blends studied here, both PLA and PBS contain methylene groups, methine group, carbonyl groups and hydroxyl group. Hence, dipole-dipole interaction and hydrogen bond (H-bond) can be formed in the blends and they can be detectable by FTIR. The main assignments are listed in Table 1. The FTIR spectrum of the PLA/PBS/Fatty acid of was classified into five regions, which corresponds to the following peaks band assignment: -C-H (in CH3) stretching (2994, 2879 cm-1), -C-H stretching (in CH2) (2945, 2858 cm-1), -C=O ester carbonyl (1700 -1780 cm-1), -C-H- deformation (1451, 1383 and 1358 cm-1), -C-O-C- stretching (1210, 1180, 1129, 1082 and 1042 cm-1) and -C-C- stretching (867 cm-1).

The assignments of functional groups in PLA/PBS/Fatty acid blends.
2. Infrared spectra of the C=O absorption in the PLA/PBS blends containing fatty acids prepared from film casting and melt blending method.
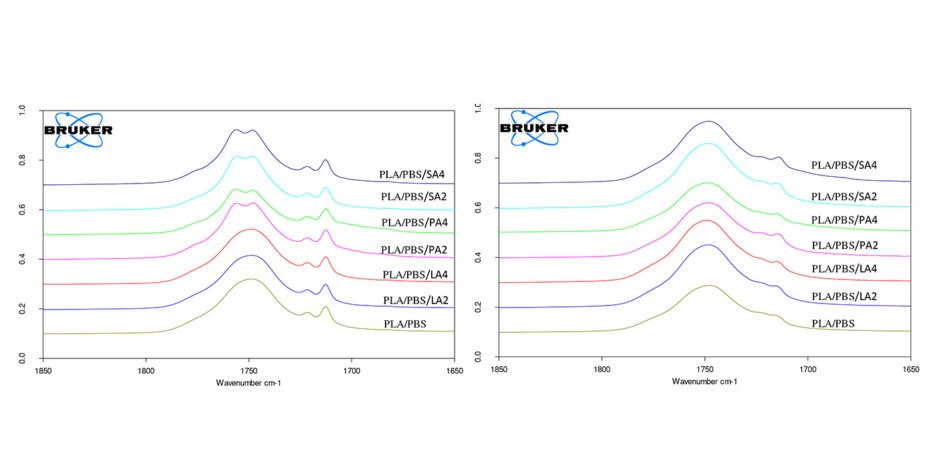
The FTIR spectra of PLA/PBS blends containing different types and amounts of fatty acids were observed. The spectra of PLA/PBS blends containing different amounts and types of fatty acids prepared from film casting and melt blending method were shown in Figure 1 and Figure 2, respectively. It shows the position of the peak, peak widths at half height and percentage integral absorbance of the component of C=O bands are similar as the PLA/PBS blends. When fatty acid was incorporated in the blend, it would be inserted between PLA and PBS chains. The interaction between -CH2- and –COOH of fatty acid interact with the chain of PLA and PBS. This causes the change in the spectrum of C=O bands. At the free C=O peak in PLA phase (ruffly 1757 cm-1), the percentage absorbance increases in 2% of fatty acid and decreases in 4% of fatty acid when compare with PLA/PBS blend. As expected, these changes are in opposite way for the band at the bonded C=O peak of PLA (ruffly 1745 cm-1). The percentage absorbance decreases in 2% of fatty acid and increases in 4% of fatty acid. This might indicate that when adding 2% of fatty acid to the blend, there are some C=O groups separated from each other. When adding 4% of fatty acid, this also cause the separation of C=O groups but the effect of –CH2 and – COOH (of acid) and -OH, -CH, -CH3 or –COOH (of polymer) show strongly effect on the change of C=O band.
From the spectrum in the range of 1650 – 1850 cm-1, it is clear that the interactions of PLA, PBS and fatty acid prepared from 2 different methods are different, especially in the blends with stearic acid and palmitic acid. In C=O band, there is the partial overlapping of the spectra. To quantify the in interaction for the blends, it should be analyzed using curve fitting of ATR-FTIR absorption spectra. In order to separate them, the curve fitting should performed using the curve analysis program of the Bruker software OPUS 7.0 to clarify the position, width and integral of each spectrum. However, this stage will be the further work to extend the knowledge of the interaction.

The spectra of PLA/PBS blends prepared from film casting method.

The spectra of PLA/PBS blends prepared from melt blend method.
Conclusion
Fourier transform infrared spectroscopy is one of the most powerful tools to provide information regarding the formation, weakening, breaking and redistribution of interaction. It is well-known that, if there are interactions between each polymer occur, the IR spectrum of the blend will be changed with respect to the coaddition of each component spectrum. Moreover, the samples prepared from different methods also show the different interactions.
Project Members:
Dr Runglawan Somsunan
Department of Chemistry, Faculty of Science, Chiang Mai University, Thailand
r.somsunan@gmail.com
Ms. Natthakan Ratsameetammajak
Department of Chemistry, Faculty of Science, Chiang Mai University, Thailand
natthakan.ntr@gmail.com
Ass.-Prof. Dipl.-Ing. Dr. Dieter Baurecht
Institute for Physical Chemistry, University of Vienna, Austria
dieter.baurecht@univie.ac.at
Project Details
- Date Februar 13, 2017
- Tags Chemistry

Comments are closed.